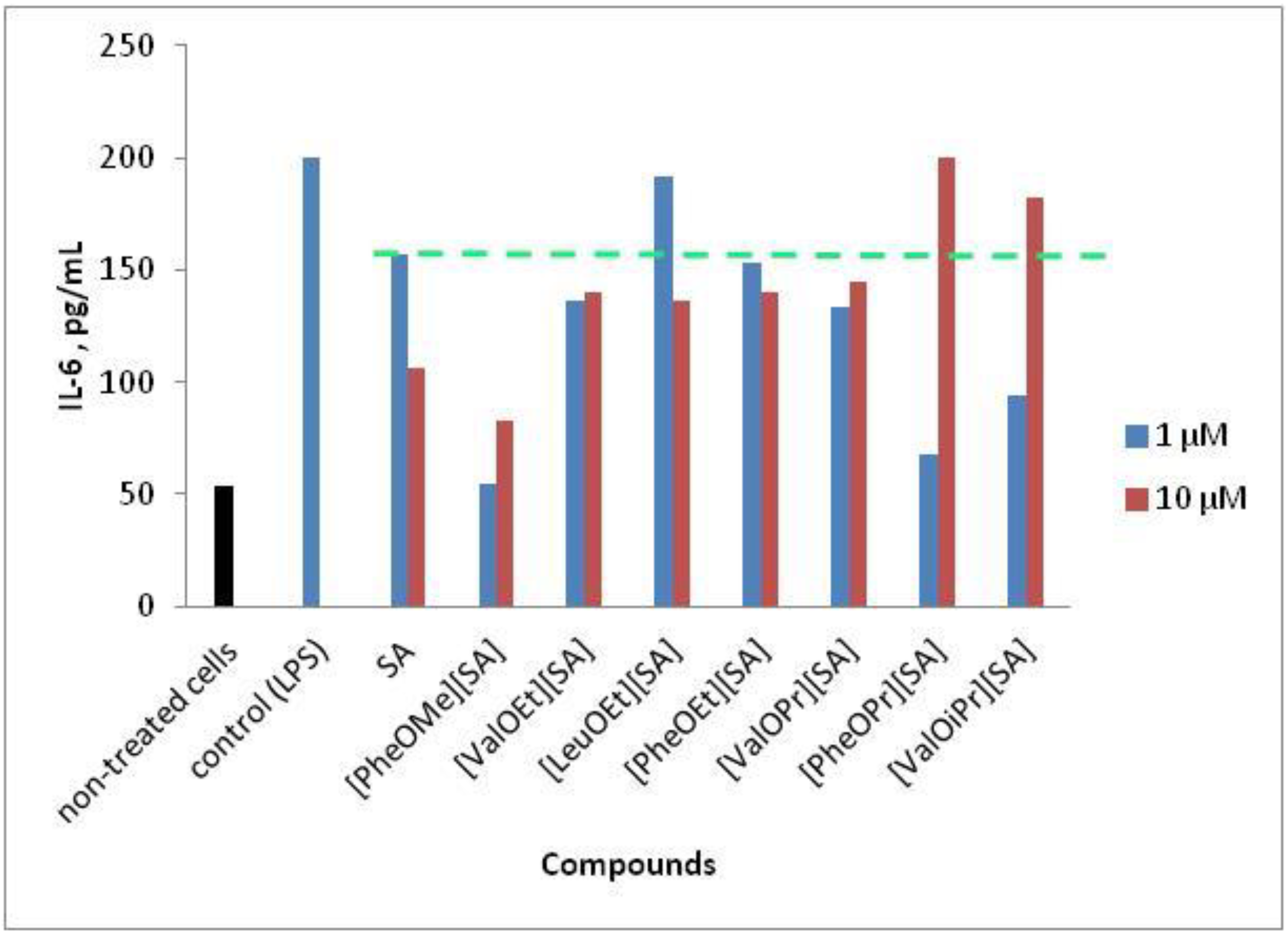
Thermodynamic study of the interactions of salicylic acid and granular activated carbon in solution at different pHs - Valentina Bernal, Liliana Giraldo, Juan C Moreno-Piraján, 2018

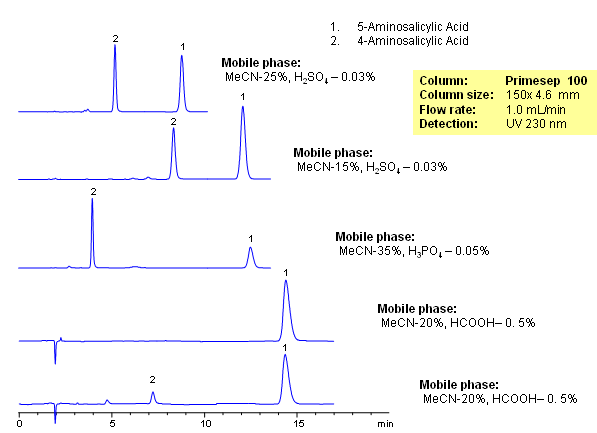
Salicylic acid, acetylsalicylic acid, methyl salicylate, salicylamide, and sodium salicylate in supercritical carbon dioxide: Solute – cosolvent hydrogen bonds formation - ScienceDirect
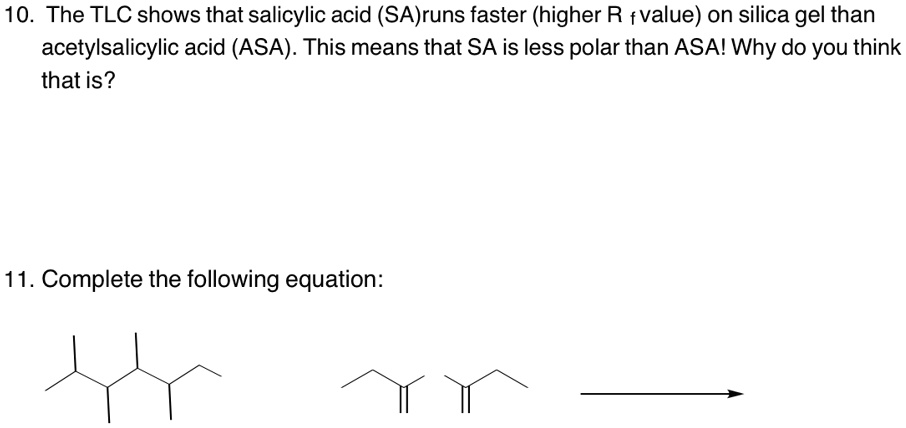
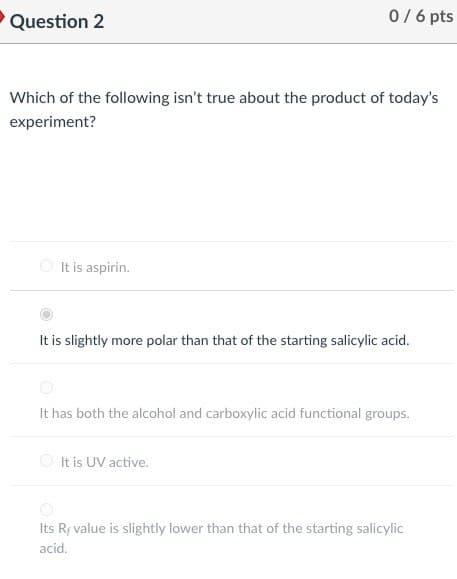
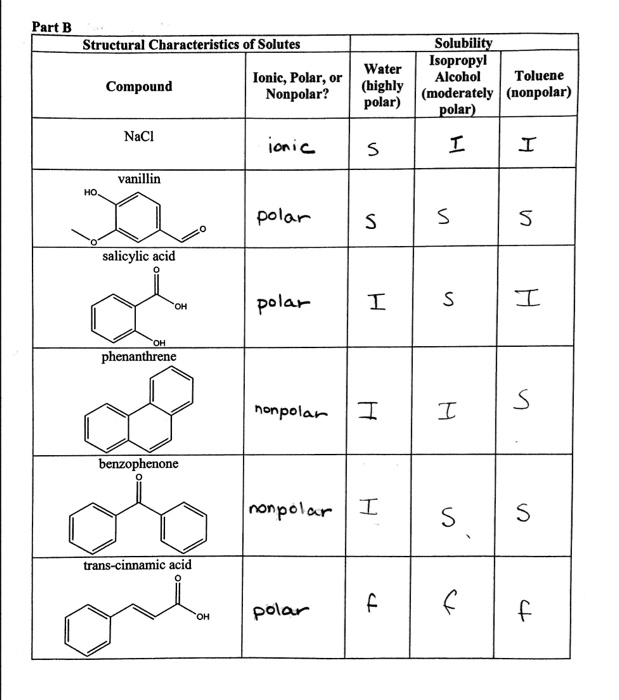
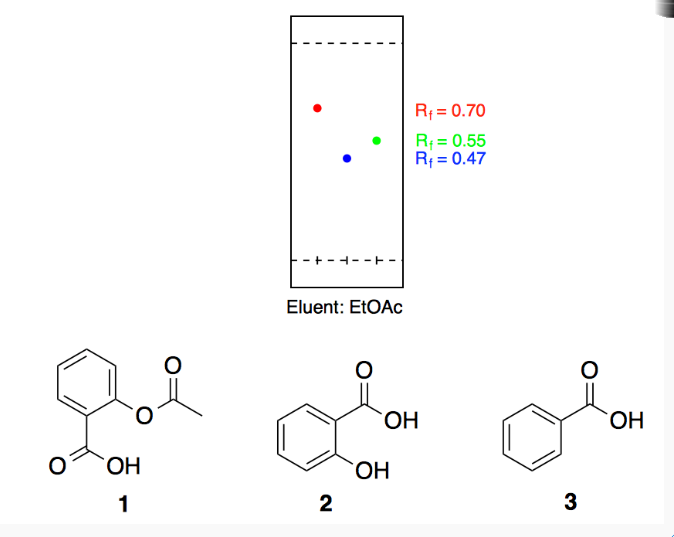
SOLVED: 10. The TLC shows that salicylic acid (SAJruns faster (higher R f value) on silica gel than acetylsalicylic acid (ASA): This means that SA is less polar than ASA! Why do

Salicylic Acid Nitration by Means of Nitric Acid/Acetic Acid System: Chemical and Kinetic Characterization | Organic Process Research & Development